xef4 structure|xef4 lone pairs : Tagatay It is the process where the orbitals of an atom fuse and form a new hybridized orbital to create the geometry of molecules along with . Tingnan ang higit pa Zynga Poker prides itself on being a fair and trusted gaming platform, which is why the card dealing algorithm, or Random Number Generator (RNG), utilized in our game is Gaming Labs Certified by Gaming .
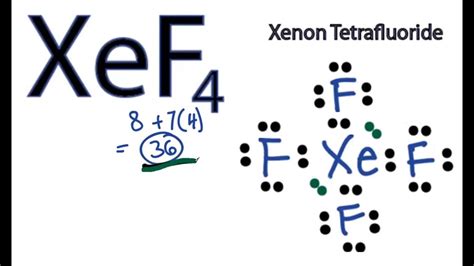
xef4 structure,Learn how to draw the Lewis structure of xenon tetrafluoride, a square planar compound with two lone pairs on xenon. Find out its molecular geometry, hybridization, and MO diagram based on VSEPR theory and quantum mechanics. Tingnan ang higit paTo begin with the Lewis structure of the compound xenon tetrafluoride, it is quite essential to know the meaning of the same. It is a simplified symbolic representation of the electrons which lie in the valence shell of a molecule. This is generally . Tingnan ang higit paThe geometry of molecules, which is also commonly known as molecular structure, is a 3-D structure of the entire molecule. It is a useful concept to understand and analyze . Tingnan ang higit paThe molecule XeF4 is a nonpolar molecule. As the geometrical structure of XeF4 is symmetric ie; square planar. All the . Tingnan ang higit paIt is the process where the orbitals of an atom fuse and form a new hybridized orbital to create the geometry of molecules along with . Tingnan ang higit paxef4 structureXenon tetrafluoride is a chemical compound with chemical formula XeF 4. It was the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon with fluorine: Xe + 2 F 2 → XeF 4This reaction is exothermic, releasing an energy of 251 kJ/mol. Learn about the structure and properties of Xef4, a binary compound of Xenon and Fluorine. Find out its valence electrons, .
An explanation of the molecular geometry for the XeF4 (Xenon tetrafluroide) including a description of the XeF4 bond angles. The electron geometry for the Xenon .
A step-by-step explanation of how to draw the XeF4 Lewis Dot Structure (Xeon Tetrafluoride).For the XeF4 structure use the periodic table to find the total n. Xenon tetrafluoride is a chemical compound with chemical formula XeF 4. It was the first discovered binary compound of a noble gas. It is produced by the chemical .
Lewis Structure for XeF4. Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and how it might .In XeF 4 (Xenon tetrafluoride) lewis structure, there are four sigma bonds and two lone pairs around xenon atom. Each fluorine atom has three lone pairs. In this tutorial, we .

Square planar structure of Xenon Tetrafluoride. XeF 4 is a planar molecule. Valence bond representation of XeF 4 may be explained if, two electrons from 5p orbitals are promoted . XeF 4 (xenon tetrafluoride) has one xenon atom and four fluorine atoms. In the XeF 4 Lewis structure, there are four single bonds around the xenon atom, with four .
The Lewis structure for XeF4 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. Remember that Xenon can have more than 8 valence electrons. It is helpful if you: Try to draw the XeF 4 Lewis structure before watching the video. Steps of drawing XeF4 lewis structure Step 1: Find the total valence electrons in XeF4 molecule. In order to find the total valence electrons in XeF4 (xenon tetrafluoride) molecule, first of all you should . Xenon tetrafluoride (XeF4) Lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge, hybridization. XeF 4 is the chemical formula for xenon tetrafluoride, the .
Verified by Toppr. The structure of XeF 4 is shown below. The central Xe atom has 2 lone pairs and 4 bond pairs of electrons. The electron pair geometry is octahedral and molecular geometry is square planar. Xe atom undergoes sp3d2 hybridisation. Xenon tetrafluoride (XeF4) is a square planar, non-polar molecule. The Xenon atom has 4 bonding pairs of electrons and 2 lone (non-bonding) pairs of electro.Lewis Structure of XeF4. Now that we know the valence electrons of Xenon Tetrafluoride, sketching its Lewis structure will be much easier. Lewis dot structure shows the relationship between valence electrons surrounding specific atoms in a molecule. Lines denote the bonds in the structure, whereas dots denote the electrons not engaged in .Square planar structure of Xenon Tetrafluoride . XeF 4 is a planar molecule. Valence bond representation of XeF 4 may be explained if, two electrons from 5p orbitals are promoted to 5d orbital. One 5s, three 5p and two 5d atomic orbitals of xenon hybridize to give six sp 3 d 2 hybridized orbitals. The four singly occupied hybridized orbitals are .xef4 lone pairs Here’s how you can easily draw the XeF 4 Lewis structure step by step: #1 Draw a rough skeleton structure. #2 Mention lone pairs on the atoms. #3 If needed, mention formal charges on the atoms. Now, let’s take .
The Lewis structure of XeF4 looks like this: Xenon (Xe) sits in the middle, and it's connected to four Fluorine atoms (F) with single bonds. Xenon (Xe) has two lone pairs, and each Fluorine atom (F) has three lone pairs. Remember that Lewis structures primarily show the bonding and valence electron distribution in molecules, and.The (twin) composition plane as an extended defect and structure-building entity in crystals. Progress in Solid State Chemistry 1979, 12 (3-4 . Coriolis coupling coefficients, generalized mean square amplitudes of vibration and shrinkage constants of XeF4 and XeOF4. Acta Physica Academiae Scientiarum Hungaricae 1968, 24 (2-3 .
Learn to determine if XeF4 is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structure and then us. I quickly take you through how to draw the Lewis Structure of XeF4 (Xenon TetraFluoride). I also go over hybridization, shape and bond angle.

Hello Guys!Today we are going to look at the Lewis Structure of XeF4 ( Xenon Tetrafluoride )Although Xenon is a noble gas it reacts with four Fluorine atoms .Symmetry, Spectroscopy, and the Molecular Structure of XeF4 The infrared spectrum of XeF4 has absorptions at 161, 291, and 586 cm-1 (two bends, one stretch), while the Raman spectrum has peaks at 218, 524, and 554 cm-1 (one bend, two stretches). Is its molecular structure tetrahedral or square planar? References: J. Am. Chem. Soc.XeF4の電子配置は八面体であり、四フッ化キセノンの分子形状は正方形の平面となる。 XeF4 Bond angles . F-Xe-F の結合角度は90度、ローンペアは180度です。 フッ素原子が互いに90度ずれているため、分子平面内で電子が対称に分布していることになります。 これ .
O XeF4 ou Tetrafluoreto de Xénon é um composto químico feito de átomos de Xénon e Fluoreto. É o primeiro composto binário do mundo a ser descoberto. É um tipo de gás nobre com a equação química de. Xe +2 F2 -> XeF4. O XeF4 tem uma aparência branca sólida e tem uma densidade de 4,040 g cm-3 numa forma sólida. Em condições . XeF4의 루이스 구조. XeF4 Lewis structure. XeF4 hybridization of central atom ----- 참고: 중심원자의 혼성오비탈 Chemistry tutorial for the Lewis dot structure and molecular geometry of xenon tetrafluoride (XeF4).
xef4 structure|xef4 lone pairs
PH0 · xef4 lone pairs
PH1 · xef4 dot and cross diagram
PH2 · xef4 dipole moment
PH3 · the shape of xef4 is
PH4 · lewis structure of xef4
PH5 · lewis dot structure xef4
PH6 · hybridization of xe in xef4
PH7 · Iba pa